Abstract
Background Laboratory diagnosis of the antiphospholipid antibody syndrome (APS) requires evidence of persistently positive lupus anticoagulant (LAC) activity in clot-based assays and/or the presence of medium-high titer anticardiolipin (aCL) and/or anti-beta 2 glycoprotein I (aB2GPI) antibodies. Although aCL and aB2GPI methodologies provide quantitative information about the antibody titer, LAC testing is interpreted in a qualitative fashion (presence versus absence).
Objectives In this study, we describe a method for measuring the LAC titer and evaluate the relationship between the LAC titer, other antiphospholipid antibodies and clinical outcomes.
Methods Residual plasma from 51 LAC-positive samples (50 unique patients) were included in this study. To estimate LAC-titers, samples were tested undiluted and subjected to serial (dependent) twofold dilutions in pooled normal plasma (PrecisionBiologic) up to a final dilution of 1:128, as appropriate. The dilute Russell's viper venom screening time (DRVVT screen, LA Check, PrecisionBiologic) and activated partial thromboplastin screening time (APTT, SynthasIL, Werfen) were measured for each dilution. A four-parameter logistic model of the dilution factor versus the clotting time was used to interpolate the LAC-titer at the upper limit of normal for both assays (APTT of 37s and DRVVT screen ratio of 1.2). Measurements of aCL, aB2GPI and anti-phosphatidylserine/prothrombin antibodies (aPS/PT) were performed using ELISA methodologies (QUANTA Lite, Werfen). Available clinical data were extracted from the electronic medical record. Continuity corrected chi-square test was used as a categorical test of association and quantile regression was used to compare medians for interval/ratio data across subgroups (Stata v17).
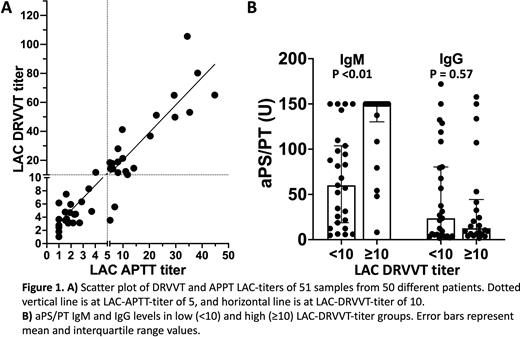
Results There was a strong positive linear relationship between the APTT and DRVVT titers (Pearson's correlation coefficient, r=0.92, Figure 1A). There was only a moderate relationship between the APTT titer and the APTT screen in the undiluted sample (r=0.50) and almost no relationship between the DRVVT titer and the DRVVT screen ratio or DRVVT screen/confirm ratio in the undiluted sample (r=0.06 and r=0.13, respectively). Data-driven partitioning identified two DRVVT titer groups: low-titer (<10) and high-titer (≥10). Empirical cutpoint estimation (Youden) identified an APTT titer threshold of 5 for predicting the DRVVT titer group. Among patients with available clinical history, 15 of 25 (60%) low-DRVVT-titer and 16 of 20 (80%) high-DRVVT-titer patients had thromboembolic/obstetric events (p=0.26). Autoimmune diagnoses were more prevalent in the low-DRVVT-titer group (19 of 25 patients, 13 with SLE) than the high-DRVVT-titer group (8 of 20 patients, 3 with SLE, p=0.03). Comparing the low and high-DRVVT-titer groups, median aCL IgM (p=0.27), aCL IgG (p=0.99), aB2GPI IgM (p=0.72) and aB2GPI IgG (p=1.00) titers were not different. High-DRVVT-titer samples had a significantly higher median aPS/PT IgM compared to low-DRVVT-titer samples (p <0.01, Figure 1B) but there was no difference observed for IgG aPS/PT (p=0.57, Figure 1B).
Conclusions APTT and DRVVT screening times are not strongly correlated with the corresponding APTT or DRVVT LAC-titer and may not indicate LAC potency. High DRVVT LAC-titers were strongly associated with IgM aPS/PT levels, but not with aCL, aB2GPI or IgG aPS/PT levels. Further research is required to evaluate the association between the LAC-titer and recurrent thrombosis in APS.
Disclosures
Pruthi:Instrumentation Laboratory: Honoraria, Membership on an entity's Board of Directors or advisory committees; HEMA Biologics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer Healthcare AG: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech Inc: Honoraria, Membership on an entity's Board of Directors or advisory committees; CSL Behring: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal